OBJECTIVES
- To study the effect of different composition of base on the physical characteristic of suppositories.
INTRODUCTIONS
Suppository is a solid formulation that has different size and
appearance and thus is suitable to be administered by rectal route. A good
suppository must be melt after being administered into the rectum and release
the drug content to achieve local or systemic effect. The drug must be spread
in a suitable base of suppository. A good base should be nontoxic, nonirritant,
no reaction with the drug and easily to be formed as a suppository. Different
composition of base will influence the rate and limit of drug release from the
suppository.
APPARATUS
- Analytical balance
- Weighing boat
- Spatula
- 50ml and 100ml beaker
- Hotplate
- 5ml measuring cylinder
- Suppository mould set
- Water bath 37oC
- Dialysis bag
- Glass rod
- 5ml pipette
- Kuvets plastics
- Spectrophotometer UV/Vis
MATERIALS
- Polyethylene glycol (PEG) 1000
- Polyethylene glycol (PEG) 6000
- Paracetamol
- Distilled water
PROCEDURE
Suppository
|
Group
|
PEG 1000
(g)
|
PEG 6000
(g)
|
Paracetamol stock solution (ml)
|
Total
(g)
|
I
|
1,5,9
|
9
|
0
|
1
|
10
|
II
|
2,6,10
|
6
|
3
|
1
|
10
|
III
|
3,7,11
|
3
|
6
|
1
|
10
|
IV
|
4,8
|
0
|
9
|
1
|
10
|
3. The suppository is
shaped using the suppository mould. The shape, texture and color of the
suppository is observed and discussed.
4. The suppository is
placed in the water bath 10ml at 37oC and the time for the
suppository to melt is recorded.
5. The suppository is
placed inside the dialysis bag and placed in the 50ml beaker. The beaker
then placed inside the water bath 37oC.
6. The sample is pipette
in 5 minutes interval and the release of the Paracetamol from the
suppository is determined using the spectrometer UV/Vis. The distilled
water must be stirred first before the sample is taken.
RESULT AND DISCUSSION
1. Compare the physical characteristics of suppositories that formulated and give comment.
2. Plot a graph average time that is required to melt the suppository against different amount of PEG 6000 in formulation. Compare and discuss the results.
3. Plot a graph of UV absorption against time. Explain.
RESULT AND DISCUSSION
1. Compare the physical characteristics of suppositories that formulated and give comment.
Based on the experiment, all the suppositories have the shape of a bullet since the mould that is being used is of this shape. The quantities of PEG 1000 and PEG 6000 different for each group and this will lead to formation of suppositories with different physical characteristics.
For the formulation that has the highest amount of PEG 1000, which means that it has the lowest quantity of PEG 6000 shows the greasiest surface compared to the other suppositories. As for the formulation with lowest amount of PEG 1000 and highest in the amount of PEG 6000, the suppository formed is the hardest of all. The former suppository provide ease to be administered to the patient compared to the latter one and the latter one may lead to pain in the process of administering.
In other words, the higher the quantity of PEG 6000 in a formulation, the less greasy the suppository will be. As for hardness, we can conclude that the higher quantity of PEG 1000 (lower quantity of PEG 6000), will produce softer suppository. As for the colour of suppositories, since the active ingredient that we used is paracetamol which is white in colour, the colour of the suppositories produce will be white but differ in the transparency degree. The formulation with the lowest amount of PEG 1000 is more transparent compared to the others.
2. Plot a graph average time that is required to melt the suppository against different amount of PEG 6000 in formulation. Compare and discuss the results.
Amount of PEG 6000
(g)
|
0
|
3
|
6
|
9
|
Average time (min)
(x +- SD)
|
36.49+-3.96
|
42.78+- 6.22
|
39.68+-6.68
|
37.19+-6.13
|
Base on the experiment, we are using two types of bases which
are polyethylene (PEG) 1000 and polyethylene (PEG) 6000. From the graph obtain
we can observed that the amount of the (PEG) use is not directly proportional
with the time for the suppository to melt. Theoretically, the higher the amount
of the (PEG) used, faster will be the suppository melt. From the experiment
that had been done, it shows that the higher the amount of (PEG), the shorter
the time taken for the suppository to melt. So from the result obtain, it show
that when the amount of (PEG) is 3g the time to melt is the slowest then the
time taken is decrease when the amount of the (PEG) is increased. It is
acceptable with the actual theory, however, for the suppository with absent of
(PEG) in the experiment, it show the lowest time for melt. Supposedly, it must
take the highest time for melt because it did not contain any (PEG) as
co-solvent. We assume that there must be some errors while handling the
experiment especially during compounding the suppository. Besides, error is due
to the temperature of the water bath that we used. Due to different group are
handling different amount of PEG, the temperature of the water bath used must
be not constant because we are using different water bath. So, the water bath
that was used should be constant so that we can get the exact results.
3. Plot a graph of UV absorption against time. Explain.
TIME
(min)
|
Average UV absorption 520nm
|
0
|
0.001
|
5
|
0.018
|
10
|
0.090
|
15
|
0.039
|
20
|
0.105
|
25
|
0.096
|
30
|
0.099
|
35
|
0.088
|
40
|
0.048
|
45
|
0.018
|
50
|
0.052
|
55
|
0.051
|
60
|
0.051
|
During the
experiment, we carry out in vitro test about the release of paracetamol
suppositories against time by using average UV absorption as indicator.
One suppository was put inside the dialysis bag which immersed into the beaker
containing distilled water maintained at 37ºC, which is equal to our body
temperature.The dialysis bag represents human biological membrane while
distilled water represents human blood plasma. The distilled water will enter
into the dialysis bag due to its higher water concentration and suppository
begin to dissolve slowly.The paracetamol then flow out through the dialysis bag into
distilled water and its amount can be detected using UV
spectrophotometer.
Our
formulation of the suppositories use
mixture of 6 polyethylene glycol 1000 :
3 polyethylene glycole 6000.The composition of Polyethylene glycole
plays an important rule in influencing the rate of melting of suppository once
it insert through rectum. The greater the average molecular weights than 1000
, more hardness the waxlike white solids
form and give different melting ranges such as for PEG 1000 is 37’C-40’C and
PEG 6000 is 56’C- 63’C.
Theoritically, the graph should be in sigmoid shape
which indicates constant drug release rate until equilibrium is achieved.Based
on the result, our graph
showing gradually increase in uv absorption which indicates that the
drug is releasing slowly for PEG formulation but
however it decrease slighly and increase back until the constant values achieved
at the end of 60 minutes. Our graph deviates from the theory may be because of
errors that occurred when we conduct the experiment. The first error we
detected is uneven heating of water bath due to the high frequency of removing the cover of hot plate
makes the temperature of water does not maintain at 37ºC which will
lead to inconstant drug release rate from the suppository. Moreover, some of
water in water bath had incidentally spill into the beaker making the volume
increases and affect the concentration of paracetamol. Besides that,distilled
water in which the dialysis bag is exposed to may not be stirred evenly before
it is taken to be tested on UV spectrophotometer.
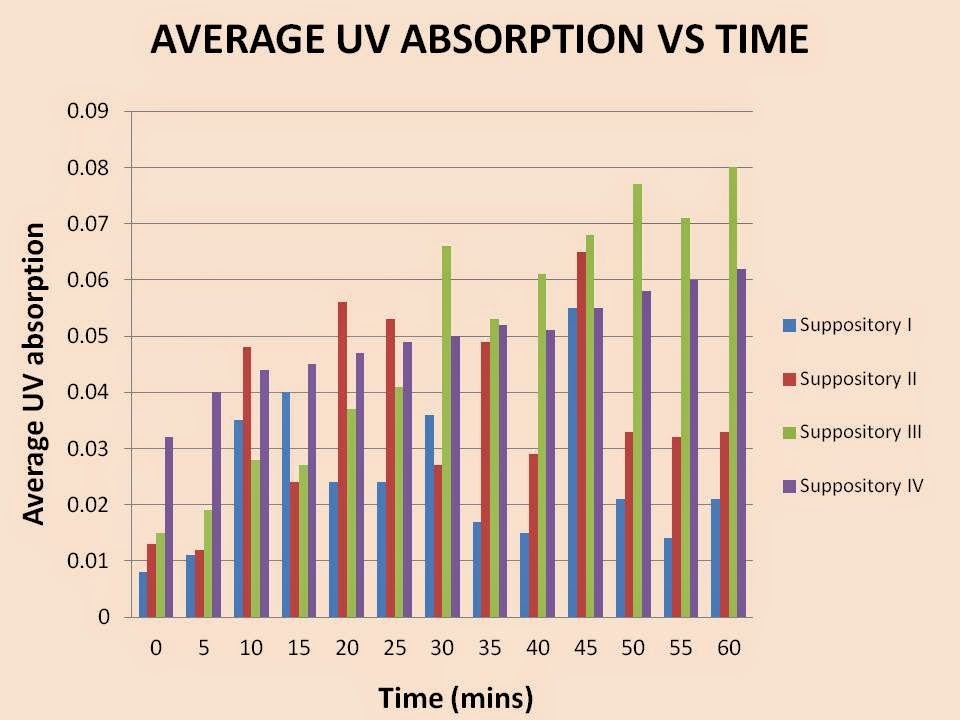
4. Plot
a graph of UV absorption for different composition of suppository formulations
against time. Compare and explain the results.
Time (min)
|
Average UV absorption at 520 nm ( x ± SD )
|
||||||||||||||
0
|
5
|
10
|
15
|
20
|
25
|
30
|
35
|
40
|
45
|
50
|
55
|
60
|
|||
UV absorption at 520nm
|
I
|
Group 1
|
0.003
|
0.006
|
0.053
|
0.054
|
0.028
|
0.029
|
0.050
|
0.012
|
0.008
|
0.012
|
0.022
|
0.005
|
0.018
|
Group 5
|
0.012
|
0.016
|
0.016
|
0.026
|
0.019
|
0.018
|
0.021
|
0.021
|
0.021
|
0.097
|
0.020
|
0.022
|
0.024
|
||
Average
|
0.008
|
0.011
|
0.035
|
0.040
|
0.024
|
0.024
|
0.036
|
0.017
|
0.015
|
0.055
|
0.021
|
0.014
|
0.021
|
||
SD
|
0.0064
|
0.0071
|
0.0262
|
0.0198
|
0.0064
|
0.0078
|
0.0205
|
0.0064
|
0.0092
|
0.0601
|
0.0014
|
0.0120
|
0.0042
|
||
II
|
Group 2
|
0.001
|
0.018
|
0.090
|
0.039
|
0.105
|
0.096
|
0.044
|
0.088
|
0.048
|
0.098
|
0.052
|
0.051
|
0.051
|
|
Group 6
|
0.025
|
0.006
|
0.006
|
0.008
|
0.007
|
0.010
|
0.010
|
0.009
|
0.009
|
0.032
|
0.013
|
0.012
|
0.015
|
||
Average
|
0.013
|
0.012
|
0.048
|
0.024
|
0.056
|
0.053
|
0.027
|
0.049
|
0.029
|
0.065
|
0.033
|
0.032
|
0.033
|
||
SD
|
0.0170
|
0.0085
|
0.0594
|
0.0219
|
0.0693
|
0.0608
|
0.0240
|
0.0559
|
0.0276
|
0.0467
|
0.0276
|
0.0276
|
0.0255
|
||
III
|
Group 3
|
0.026
|
0.032
|
0.029
|
0.024
|
0.032
|
0.037
|
0.067
|
0.042
|
0.047
|
0.052
|
0.055
|
0.058
|
0.060
|
|
Group 7
|
0.004
|
0.006
|
0.026
|
0.030
|
0.042
|
0.045
|
0.064
|
0.063
|
0.075
|
0.083
|
0.099
|
0.083
|
0.100
|
||
Average
|
0.015
|
0.019
|
0.028
|
0.027
|
0.037
|
0.041
|
0.066
|
0.053
|
0.061
|
0.068
|
0.077
|
0.071
|
0.080
|
||
SD
|
0.0156
|
0.0184
|
0.0021
|
0.0042
|
0.0071
|
0.0057
|
0.0021
|
0.0148
|
0.0198
|
0.0219
|
0.0311
|
0.0177
|
0.0283
|
||
IV
|
Group 4
|
0.060
|
0.075
|
0.080
|
0.084
|
0.087
|
0.091
|
0.093
|
0.095
|
0.094
|
0.102
|
0.106
|
0.107
|
0.109
|
|
Group 8
|
0.004
|
0.005
|
0.007
|
0.005
|
0.006
|
0.006
|
0.006
|
0.009
|
0.007
|
0.007
|
0.009
|
0.012
|
0.014
|
||
Average
|
0.032
|
0.040
|
0.044
|
0.045
|
0.047
|
0.049
|
0.050
|
0.052
|
0.051
|
0.055
|
0.058
|
0.060
|
0.062
|
||
SD
|
0.0396
|
0.0495
|
0.0516
|
0.0559
|
0.0573
|
0.0601
|
0.0615
|
0.0608
|
0.0615
|
0.0672
|
0.0686
|
0.0672
|
0.0672
|
||
Different suppository
base compositions will have different effects on the drug release rate over
time. In this experiment, we varied the ratio amount of PEG 1000 and PEG 6000
in each of the suppository. From suppository
I to suppository IV, the average UV absorbance should increase all the time
following this order as the amount of PEG 1000 decreasing while amount of PEG
6000 increasing in this order.
Suppository I has the
highest amount of PEG 1000 and none PEG 6000. According to the theory, it
should be showing the highest drug releasing rate as well as the UV absorption.
This is due to lesser hydroxyl group present in PEG 1000. Thus, there are less
strong hydrogen bond formed between the molecules of PEG 1000 and molecules of
paracetamol. Little energy would be needed to break the bonds to release the
drug. Hence, it would have a higher releasing rate. Higher releasing rate would
cause the UV absorption to be higher.
On the other hand,
Suppository IV has the highest amount of PEG 6000 with none PEG 1000. It has
more hydroxyl groups present in it. Thus, there are more hydrogen bonding
formed between the molecules of PEG 6000 and the molecules of the paracetamol
when they are mix together and formed a suppository. It has the highest
molecular weight. Highest molecular weight would cause a lower drug releasing
rate. Therefore, a longer time and higher energy is needed in order to break
down the strong hydrogen bond and subsequently release the drug. This will
result in longer drug releasing time and lower the drug releasing rate. This
will further lower the UV absorption.
Suppository II and
Suppository III has intermediate UV absorption. Suppository II has higher UV
absorption than Suppository III as Suppository II has higher PEG 1000 but lower
PEG 6000 than Suppository III. Thus, there are less hydrogen bond formed
between the Suppository II bases and paracetamol. Less energy would be needed
to break the bonds to release the drug. Hence, it would have a higher releasing
rate than Suppository III. Higher releasing rate would cause the UV absorption
of Suppository II to be higher than Suppository III.
However,
according to our graph, we can see that Suppository III has the highest UV absorption,
while, Suppository I has lowest UV absorption rate, which indicates that it has
lowest drug releasing rate. Meanwhile, Suppository II and Suppository IV show
intermediate UV absorption. This did not comply with the actual theory. Besides
that, the graph showed deviated much in comparison between results of each two
groups. It is due to many errors arisen in the experiment. These errors can be
due to the improper method of compounding, non-homogenous dispersion of
Paracetamol in the PEG suppository base, inaccuracy of the UV spectrometer, and
so on. Other errors that may occur include inconsistency in temperature which
may affect the drug release from the suppository, the sample in the beaker did
not stir well before pipette out from the solution or could be due to dirt or
impurities present in the cuvettes which are used to fill the sample and hence
lead to deviation of the result.
5. What is the function of each
ingredient that is used in the preparation of these suppositories? How does the
usage of different content of PEG 1000 and PEG 6000 affect the physical
properties of suppository formulation and rate of releasing of drug from it?
PEG
6000 act as suppository bases.The Paracetamol that is used in the
preparation of the suppository acts as the active ingredient. It is the main
substance in the drug formulation which has the major role in contributing to
the required drug therapeutic effects in the body.
The
use of different contents of PEG 1000 and PEG 6000 results in different effects
on the physical characteristics, subsequently affecting the rate of drug
released from the suppository. More hydrogen bonds are formed between the PEG
6000 molecules and drug molecules when the more PEG 6000 is used. This will
result in the increase of the hardness of the suppository and also the
difficulty of the drug released from the suppository. Besides that, the
production of dry, hard, smooth, coarse, tackier and clear white suppository
will be obtained.
Hence,
it is important to choose the correct combination ratio of PEG 1000 and PEG
6000 to avoid the production of extremely hard or soft suppository and to
ensure an optimum bioavailability of the drugs can be obtained.
As
the proportion of PEG6000 increases, the drug becomes more difficult to be
released from the suppository.
CONCLUSION
Different percentage of combination of PEG
1000 and PEG 6000 affects the physical characteristics of the suppository and
the rate of release of the active ingredient.
REFERENCES
- https://www.inkling.com/read/ansel-pharmaceutical-dosage-form-drug-delivery-9th/chapter-12/suppository-bases
- http://www.drugs.com/inactive/polyethylene-glycol-6000-274.html


Tiada ulasan:
Catat Ulasan